
How to Regulate the Introduction of Vaccines and Other Innovative Products
Introducing a new product in risky markets such as pharmaceuticals or aviation can result in large damages, if a drug or a plane turn out to be defective, but it can also produce high societal benefits, well beyond the individual gains for customers. In economic jargon, it can have large positive externalities, thus contributing to the general welfare.
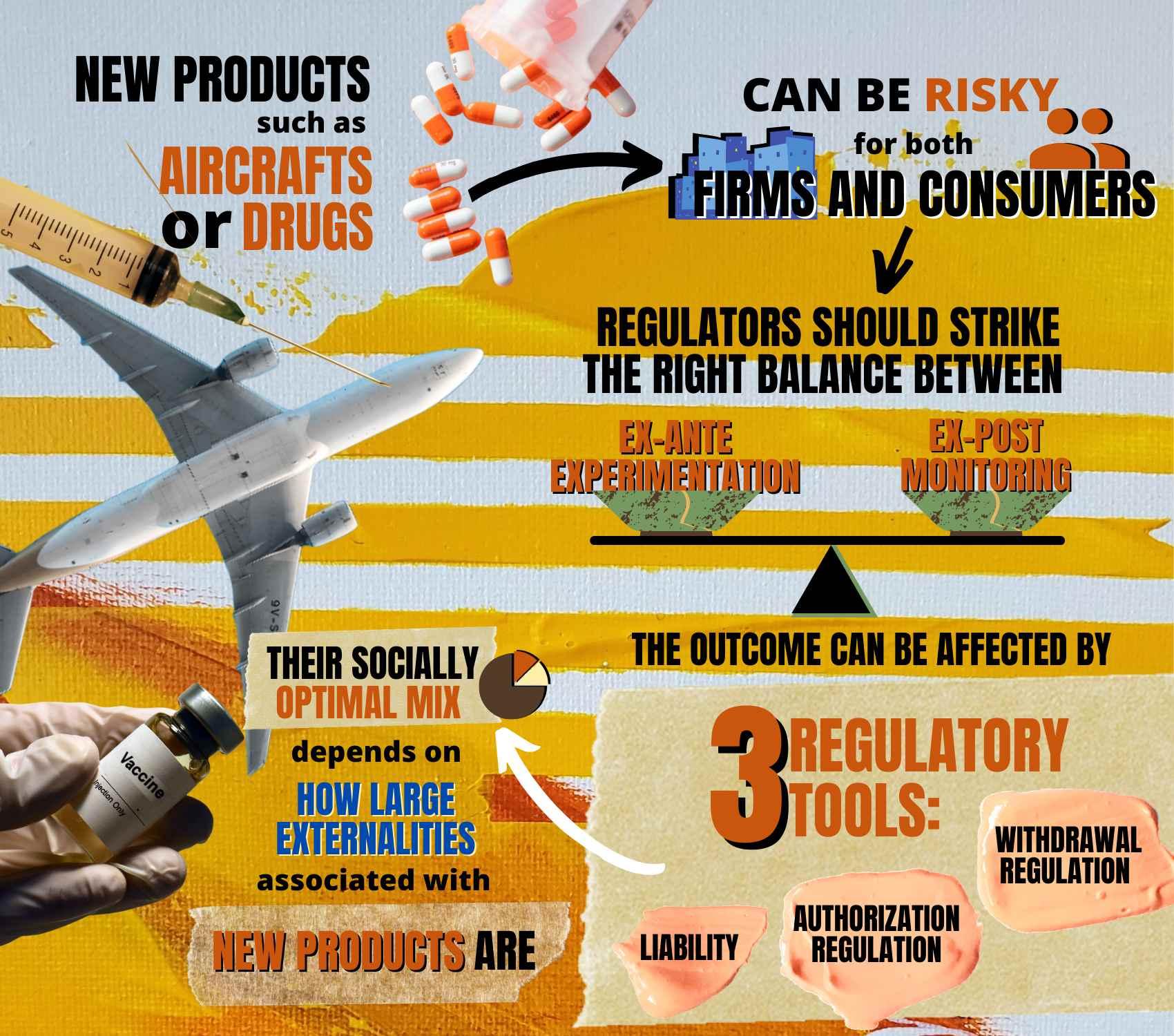
If they want to both incentivize innovation and avoid the introduction of unsafe products, regulators should strike the right balance between ex-ante experimentation, performed by firms before market introduction, and ex-post monitoring of effects on users, argue Marco Ottaviani (Bocconi Department of Economics), Emeric Henry (Sciences Po), and Marco Loseto (University of Chicago) in their latest paper, published in Management Science.
Professor Ottaviani and his co-authors design a two-phase model of information diffusion and evaluate how its outcome can be affected by three regulatory tools that evolved over the course of the 20th century: liability, withdrawal regulation and authorization regulation.
Infographics by Weiwei Chen
In the first phase, firms collect information by undertaking costly experimentation to decide whether to introduce the product or abandon experimentation. In the second phase, following adoption, monitoring activities provide new, less systematic information and the product is withdrawn if sufficiently bad news about its effects is collected.
In this model, the socially optimal mix of the three tools depends on how large externalities are.
"When we started working on this project," Professor Ottaviani says, "we did not anticipate the key features we found for the optimal mix of instruments for regulating the introduction of risky products. First, liability should be banned for firms introducing products that generate large societal benefits relative to company profits. Second, whenever they are used, regulatory instruments-such as ex-ante approval regulation based on controlled tests made before market introduction and ex-post monitoring leading to mandatory recalls on the basis of on post-marketing observational studies-should be lenient. The optimal amount of leniency avoids discouraging firms from introducing potentially valuable products in the first place."
Even if the model was developed before the pandemic, it has implications for COVID-19 drugs and vaccines. "Our analysis suggests that drug regulators should opt for a faster approval and protect innovators against liability claims, given the large positive externality associated to the control of pandemic diseases," Prof. Ottaviani concludes.
Emeric Henry, Marco Loseto, Marco Ottaviani, "Regulation with Experimentation: Ex Ante Approval, Ex Post Withdrawal, and Liability." Management Science, published online in Articles in Advance, November 29, 2021. DOI: https://doi.org/10.1287/mnsc.2021.4164.